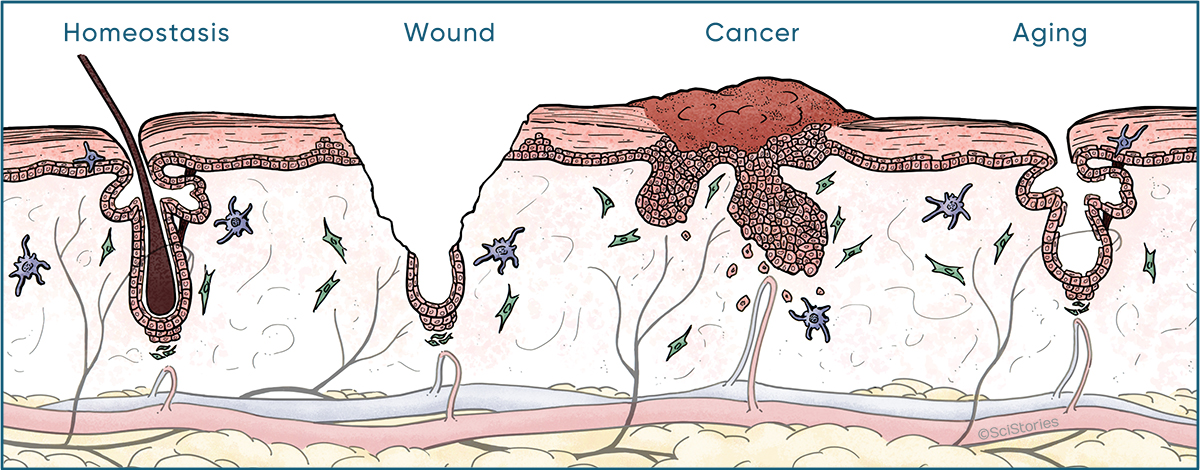
Defined by golden standards of long-term self-renewal and multi-lineage differentiation, stem cells (SCs) come in different flavors. In mammals, adult SCs are essential units to orchestrate postnatal remodeling and repair damage. In contrast to steady state, SCs in coping with stress often expand their fates and embark on behaviors distinct from their homeostatic patterns, known as plasticity. While plasticity is essential for organismal survival, its derailed regulation poses disease vulnerability to individuals, especially those undergoing prolonged stress. Under these scenarios, SCs are subjected to functional exhaustion frequently observed in aging, or malignant transformation that occurs in cancer. Research in the Ge lab applies principle of developmental biology in order to understand molecular mechanisms underlying SC plasticity, and how its deregulation leads to human diseases.
One of the key hypotheses we set out to test is this long postulated idea “cancer is a wound that never heals”. Parallels between wound repair and cancer have emerged in many contexts, begging questions in regards to their molecular origin and functional relevance. Mouse skin represents an excellent model to address these outstanding issues. Its SCs are well defined, abundant, and genetically tractable. Studying squamous cell carcinomas (SCCs) of the skin, resembling those in the head and neck, esophagus, lung and cervix which are highly deadly, we found SCs adopt a plastic phenotype so called “‘lineage infidelity”, entailing a wide spread co-expression of otherwise lineage restricted genes. Rather than a cancer specific phenomenon, lineage infidelity occurs transiently during normal wound repair and is functionally required for SCs to cope with stress. Lineage infidelity is hijacked by SCs of the SCCs where it is sustained to maintain malignancy. Mechanistically, it signifies a state where lineage genes are functionally re-wired from a homeostatic network into a stress-specific one, presumably in cooperation of stress-induced genes, at the level of chromatin and non-coding regulatory elements. Our most recent observations suggest lineage infidelity occurs across many diseases and stress conditions including aging and chronic wounds, therefore may represent a core mechanism SCs use to steer fate choices and a valuable therapeutic target in human patients.
For more details, please see Yejing’s recent talk at MDACC Enjoy Science. https://mediaplayer.mdanderson.org/video-full/9D2A4804-5FC4-40DC-97AA-82C229B3DBCE
Transposons are interspersed genomic repeats that constitute more than 40% of the mammalian genome. Most retrotransposons are either degenerated or domesticated as host regulome to orchestrate lineage gene expressions, while an extraordinary group morphed into host proteins or donated coding functions during a critical stage of mammalian development. A few rare evolutionarily young species remain virally active, causing germline mutations and modulating immune functions. Retrotransposon activities are widely observed in cancer, although the extent to which retrotransposons exert active viral form and function is unclear. Their molecular trigger is poorly defined and therapeutic potential in cancer is under-explored.
In our lab, we address these challenges by using a genetic model lacking what we have found to be a crucial epigenetic repressor of retrotransposons in adult skin. Built on the genetic and functional genomic tools we have developed in the lab, we exploit our ability to read and perturb retrotransposons in the skin. Skin is a uniquely suitable system, given its well-established, readily accessible, and highly synchronized adult stem cell populations, not only mediating homeostatic tissue remodeling and regeneration, but also responding to stress by driving wound repair and tumorigenesis. Our preliminary data revealed potent, selective reactivation of endogenous retroviruses (ERVs, a type of retrotransposons) in our model, through which we will systematically dissect the role of these selfish genes and host mechanisms restraining them in adult regenerative tissues.